Introduction Flow cytometry(FC) plays an important role in the diagnosis of hematologic diseases and the study of cell maturation. Spectral multicolor flow cytometry(SMFC) has shown an advantage over traditional FC that more fluorescent markers could be detected simultaneously, more antigen combinations could be made, and the expression of cells could be scrutinized. However, published studies focused on lymphocyte subsets and the differentiation between hematogones and B-acute lymphoblastic leukaemia/lymphoma(ALL/LBL) minimal residual disease(MRD) detection, and there are few studies on myeloid development and expression. Besides, more powerful and cutting-edge software are needed for complicated combinations from SMFC because traditional dot plots are unable to meet the demand of analysis. Here we design a one-tube 24-color panel combining with the multidimensional data analysis software to study the expression and maturation of normal and malignant myeloid cells including minus subgroups. We hope to improve the sensitivity of MRD by FC and explore more information about myeloid diseases, finally promote the development of artificial intelligence(AI) in clinical FC diagnosis.
Methods: the one-tube 24-color panel was designed according to our experience and Euroflow recommendation. it is composed of backbones including CD45 and myeloblast markers CD34, CD117 and HLA-DR, adding myeloid markers CD33, CD13, CD371, CD15, CD64, CD11c,CD14, CD36 and CD11b, routine leukaemia associated immunophenotyping(LAIP) or different from normal(DFN) markers CD4, CD19, CD7, CD2, CD56,CD96,CD123, CD38, CD200, CD71 and CD9. The control database consisted of 20 normal bone marrow(BM) specimens, including 8 healthy donors and 12 patients with other diseases that were in complete remission(CR) after treatment. To verify the effectiveness of the panel, 4 BM samples from acute myeloblastic leukaemia(AML) patients with MRD positive or relapsed status were selected, with malignant myeloblasts of 0.23% (sample A1), 4.3%, 30.31% (and 6.47% abnormal mast cells, sample A3), 0.29% (16.49% basophils) (sample A4), respectively. The data was acquired by a 3 laser 38-color Cytek spectral FC, and analyzed by Kaluza and Flowjo software. The results were compared with those of conventional 3 - laser 8 - color Canto FC.
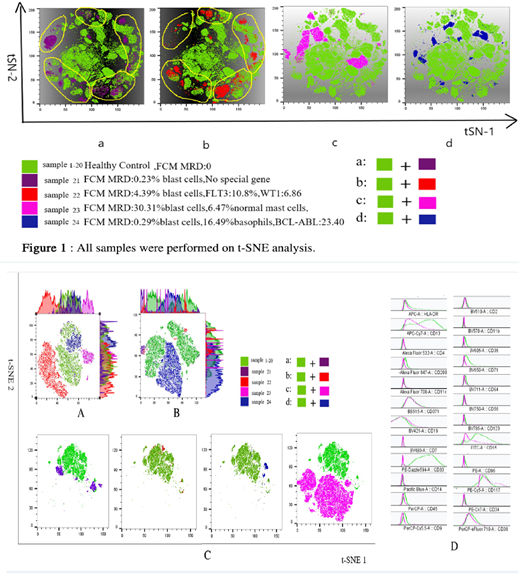
Results when analyzing the common antibodies acquired by two kinds of FCs and software, the similarities and good correlations were shown about the percentage of myeloid subsets and expression pattern of antigens, no matter in normal or abnormal specimen. However, SMFC can analyze subsets of myeloid cells more clearly and detailedly, especially in minus subgroups like mast cells or basophils. Abnormal expression of tumor cells could be clearly observed in both FCs, and more abnormalities could be found with 24 color analysis, especially mast cell abnormalities. The t-SNE plug-in in Flowjo was used to conduct dimension-reduction analysis on 20 samples (see figure), with hundreds of dot plots being merged into one multi-dimensional picture. The normal samples were similar, but the abnormal samples were different from the normal ones. However, the t-SNE pictures were same only for the specimens that malignant myeloblasts were the only abnormality. The other two samples with abnormal mast cells or basophils showed different t-SNE figures. In the process of automatic clustering, CD117 and CD34 positive myeloblasts, basophils and mast cells were classified as three clusters according to the proportion of cells, surface antigen expression, and of fluorescence intensity of antigen expression. A more obvious aggregation was formed by malignant cells than normal cells, and was positively related to tumor burden. Since mast cells and basophils were low percentages in normal specimens, the abnormal clusters were more obvious when the proportion of these two types of cells increased.
Conclusion Combined with cutting-edge software(BD FlowJO), SMFC can offer more cellular information that is unmatched by traditional FC. This study is only the preliminary attempt of our laboratory. With detailed data from more samples and longer-termed clinical trials, it would be a promising method to explore and study the myeloid maturation, improve the sensitivity of MRD, and promote the application of AI.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal